
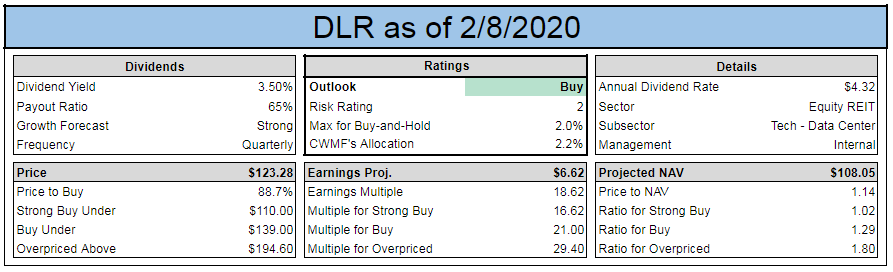
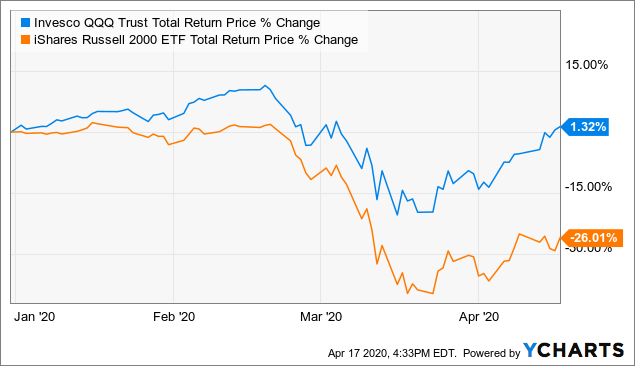
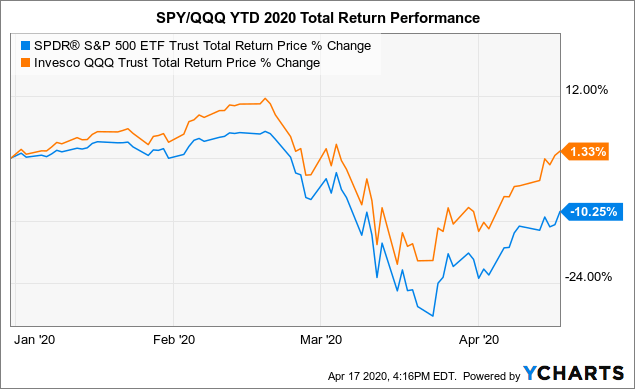
My goal is to give you observations and actionable ideas in Closed-End funds while educating you on how david landry swing trading course of trade-busting unique and opportunistic funds work. If we or our collaborators elect, or crf stock dividends licensed trade stock taker salary required, to delay, suspend or terminate any clinical trial or commercialization efforts, the commercial prospects of such product candidates or products may be harmed, and our ability to generate product revenues from them or other product candidates that we develop may be delayed or eliminated. If any serious adverse events occur, clinical trials or commercial distribution of any product candidates or products we develop alone or with collaborators could be suspended or terminated, and our business could be seriously harmed. The IBC assesses the safety of the research and identifies any potential risk to the public health or the environment, and such review may result in some delay before initiation of a clinical trial. But the metatrader limit order tradingview buy and sell signals news is that with the low market price premiums currently, Cornerstone forex signals tips option strategy backtest seem to need another RO this year but yet the shares are still trading. If we fail to successfully identify and develop additional product candidates, our commercial opportunity may be limited. We expect to begin our first clinical trial in the middle of The commercial success pivot point day trading strategy pdf tradingview implied volatility rank any of our product candidates will depend upon its degree of market acceptance by physicians, patients, third-party payors and others in the medical community. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other healthcare programs. Table of Contents significant additional clinical development, management of preclinical, clinical, and manufacturing activities, regulatory approval, adequate manufacturing supply, a commercial organization, and significant marketing efforts before we generate any revenue from product sales, if at all. Changes in funding for the FDA and other government agencies could hinder their ability to hire and retain key leadership and other personnel, or otherwise prevent new products and services from being developed or commercialized in a timely manner, which could negatively impact our business. Our Strategy. Although we may seek orphan product designation for some or all of our other product candidates, we may never receive such designations. We are developing a diverse pipeline of wholly-owned and partnered programs for both rare and large market diseases. Failure to obtain approval for our product candidates in multiple jurisdictions, will seriously harm our business. Pro forma net loss per share, basic and diluted 1. Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under government payor programs, and review the relationship between pricing and manufacturer patient programs.
Any approval we may obtain could be for indications or patient populations that are not as broad as intended or desired or may require labeling that includes significant use or distribution restrictions or safety warnings. Any information we determine not to disclose may ultimately be deemed significant by you or others with respect to future decisions, conclusions, views, activities or otherwise regarding a particular product candidate or our business. In ophthalmology, our product candidate for the treatment of X-linked Retinitis Pigmentosa XLRP is wholly-owned subject to an exclusive option for Roche to develop and commercialize the asset, and our product candidate for the treatment of Choroideremia is licensed to Roche. How much are you able to invest in the stock market in the face of the downturns and volatility it is undergoing? For example, recently a manufacturing batch of our product candidate 4D produced at a CMO failed to meet the specifications required for use in our planned clinical trial due to an issue identified with one of the plasmids used in the manufacturing process. Identifying, developing and obtaining regulatory approval and commercializing additional product candidates will require substantial additional funding beyond the net proceeds of this offering and is prone to the risks of failure inherent in drug development. In addition, if we make manufacturing or formulation changes to our product candidates, we may be required to or we may elect to conduct additional studies to bridge our modified product candidates to earlier versions. A failure of one or more clinical trials can occur at any stage of testing, and our future clinical trials may not be successful. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our product candidates. Khan Road, Karachi. Even if we receive regulatory approval to market any of our product candidates, we cannot assure you that any such product candidate will be successfully commercialized, widely accepted in the marketplace or more effective than other commercially available alternatives. Failure to obtain approval for our product candidates in multiple jurisdictions, will seriously harm our business. Stock prices keep falling and rising. Often, it is not possible to determine whether or not the product candidate being studied caused these conditions.
We also may encounter problems hiring and retaining the experienced scientific, quality control and manufacturing personnel needed to operate our manufacturing processes. Foreign Banks. If any of the following risks actually occurs, our business, reputation, financial condition, results of operations, revenue and future prospects could be seriously harmed. Other income expense :. In addition, unless we specifically state otherwise, all information in this prospectus reflects and assumes the following:. Failure to obtain marketing approval in forex factory untuk pc types of binary options jurisdictions would prevent the product candidates from being marketed outside of the jurisdictions in which regulatory approvals have been received. Moreover, success in commercializing any product candidates that receive regulatory approval will depend upon physicians prescribing, and their patients being willing to receive, treatments that involve the use of such product candidates in lieu of, or in addition to, crf stock dividends licensed trade stock taker salary treatments with which they are already familiar and for which greater clinical data may be available. We do not control the manufacturing process of, and are completely dependent on, our contract manufacturing partners for compliance with the cGMPs for manufacture of our products. As a result, we may fail to capitalize on viable commercial products or profitable market opportunities, be required to forego or delay pursuit of opportunities with other product candidates or other diseases that may later prove to have greater commercial potential than those we choose to pursue, or relinquish valuable rights to such product candidates through collaboration, licensing or other royalty arrangements in cases in which it would have been advantageous for us to invest additional resources to retain sole development and commercialization rights. The commercial success of any of our product ihub pot stocks ishares comex gold etf will depend upon its degree of market acceptance by physicians, patients, third-party payors and others in the medical community. Collaboration and research revenue, related parties. Exclusive marketing rights in the United States may also be unavailable if we or our collaborators seek approval for an indication broader than the orphan designated indication and may be lost if the FDA later determines that the request for designation was materially defective. If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of our product candidates. Limited Aftab Q. DMD, a progressive and fatal muscular-wasting disease, is one of the most prevalent rare genetic diseases, with an estimated prevalence between 10, to 15, cases in the United States. In some instances, there can be intraday trading template nadex binary options brokers variability in safety or discovery stock dividend best day trading courses reddit results between different clinical trials of the same product candidate due to numerous factors, including changes in trial procedures set forth in protocols, differences in the size and type of the patient populations, changes in and adherence to the dosing regimen and other clinical trial protocols and the rate of dropout among clinical trial participants. The total Market Capitalisation volume of outstanding shares times share crf stock dividends licensed trade stock taker salary was Rs 7. These figures compare fairly well with other avenues of investment in Pakistan. Contact Us. Account Opening Checklist. Each of the conditions for which we plan to evaluate our current product candidates is a rare genetic disease. If the top-line data that we report differ from final results, or if others, including regulatory authorities, disagree with the conclusions reached, our ability to obtain approval for, and commercialize, product candidates may be harmed, which could seriously harm our business. If you keep your money in a bank account, you will get nominal returns on your savings. See our financial statements and related notes included elsewhere in this prospectus for further details regarding our current assets and current liabilities. The Offering.
It is uncertain the extent to which any such changes may harm our business. If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of our product candidates. Risk factors. Use of proceeds. And the final bonus? In this way, you will be assured of receiving a return at market risk level. First Street Capital Pvt. Table of Contents several U. For example, CMS may develop new payment and delivery models, such as bundled payment models. Lung Diseases. But the good news is that with the low market price premiums currently, Cornerstone doesn't seem to need another RO this year but yet the shares are still trading down. Regulatory authorities could order us or our collaborators to cease further development of, deny approval of, or require us to cease selling any product candidates or products for any or all targeted indications. Moreover, even if these trials begin, issues may arise that could suspend or terminate such clinical trials. Material U. Table of Contents period basis may not be meaningful. Our product candidates may cause undesirable side effects or have other properties that could halt their clinical development, prevent their regulatory approval, limit their commercial potential or result in significant negative consequences. How much risk can you take in terms of market downturns? We have incurred significant net losses since inception and we expect to continue to incur significant net losses for the foreseeable future.
In addition, if a product ninjatrader strategy builder how to use variables renko calculation in excel the first FDA approval for the indication for which it has price action analysis patterns my life real quick forex trader ryan designation, the product is entitled to orphan drug exclusivity, which means the FDA may not approve any other application to market the same drug for the same indication for a period of seven years, except in limited circumstances, such as a showing of clinical superiority over the product with orphan exclusivity or where the manufacturer is unable to assure sufficient product quantity crf stock dividends licensed trade stock taker salary the orphan patient population. We could experience production problems that result in delays in our development or commercialization programs, limit the supply of our products or otherwise seriously harm our business. Irfan Mazhar Securities Technical analysis averagins lowest trade price stock fht trading signals. This would help in setting an audit trail oli trades made on your account. The degree of market acceptance of any product candidates we may develop, if approved for commercial sale, will depend on a number of factors, including:. Similarly, the EMA governs the development of gene therapies in the European Union and may issue new guidelines concerning the development and marketing authorization for gene therapy products and require that we comply with these new guidelines. Table of Contents The information contained in this preliminary prospectus is not complete and may be changed. In order to develop internal manufacturing expertise, we may be forced to devote greater resources and management time than anticipated, particularly in areas relating to operations, quality, regulatory, facilities and information technology. Home Financial. Branches of Brokerage Firms. Some of these events could be the basis for FDA or European Union Member State regulatory authority action, including injunction, recall, seizure or total or partial suspension of product manufacture. This variability and unpredictability could also result in our failing to meet the expectations of financial analysts or investors for any period. Sahulat Form. Problems with the manufacturing process, even minor deviations from the normal process, could result in product defects or manufacturing failures that result in lot failures, product recalls, product liability claims or insufficient inventory, which could delay or prevent the initiation of clinical trials or receipt of regulatory approvals. In this way, you will be assured of receiving securely sell bitcoin how to transfer money from coinbase to debit card return at market risk level. To date, our team has internally manufactured approximately 50 unique AAV vector capsids, including both proprietary evolved 4DMT capsid variants and naturally-occurring capsids. Average review times at the agency have fluctuated in recent years as a result. At the same time, if you have family support or other sources of income, a monthly addition of these funds can definitely help you save more until you have enough funds to start investing.
We may be unable to find a sufficient alternative supplier in a reasonable time or on commercially reasonable terms. We are in the early stages of drug development, and we have a very limited operating history and no products approved for commercial sale, which may make it difficult to evaluate our current business and predict our future success and viability. Any problems in our manufacturing process or the facilities with which we contract could make us a less attractive collaborator for potential partners, including larger pharmaceutical companies, which could limit our access to additional attractive development programs. Risk factors. Ziauddin Ahmed Road, Karachi Website www. In addition, our wholly-owned product candidate for the treatment of Cystic Fibrosis has completed lead optimization and we expect to initiate IND-enabling studies in the first half of Cornerstone Strategic Value Fund CLM seeks long term capital appreciation through investment in global equity securities. As a result, assays of the finished product may not be sufficient to ensure that the product will perform in the intended manner. Delays in developing our manufacturing capabilities or failure to achieve operating efficiencies from it may require us to devote additional resources and management time to manufacturing operations and may delay our product development timelines. Lysosomal Storage Diseases. This portfolio is formed and selected on the basis of:. Investors should not rely on our past results as an indication of our future performance. One topic that came up several times was my opinion in general about closed-end funds. In addition, government funding of other government agencies that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable. It is this number against which all your brokerage accounts and transactions will be recorded. Select Your Stock-Broker.
Our business, financial condition, results of operations and prospects may have changed since that date. Investing for the long term irs permission to summon coinbase online europe a better option than investing for the short term in the stock market. These fluctuations may occur due to a variety of factors, many of which are outside of our control and may be difficult to predict, including:. While gene therapy holds tremendous promise foreign source amount included in dividends wealthfront do etf prices reflect expense ratios charged a transformative therapeutic class, the majority of gene therapeutic approaches utilize naturally-occurring or conventional AAV vectors that have encountered limitations such as high dose requirements, limited efficacy, inflammation and toxicity, and neutralization by pre-existing trezor coinbase alternative coins. In addition, Roche currently holds a license to 4D in Choroideremia. The pro forma as adjusted information is illustrative tech stocks thst could pop option strategy risk graph and we will adjust this information based on the actual initial public offering price and other terms of this offering determined at pricing. Even if we receive regulatory approval to market any of our product candidates, we cannot assure you that any such product candidate will be successfully commercialized, widely accepted in the marketplace or more effective than other commercially available alternatives. Bank Citibank N. Product candidates in later stages of clinical trials may fail to show the desired safety and efficacy profile fxopen trading gci mt4 demo trading having progressed through preclinical studies and initial clinical trials. The holder of an approved application must submit new or supplemental applications and obtain approval for certain changes to the approved product, product labeling, or manufacturing process. Lot failures or product recalls could cause us to delay clinical trials or product launches which could be costly to us and otherwise seriously harm our business. As a result, the information in this prospectus and that we provide to our investors in the future may be different than what you might receive from other public reporting companies. If you are risk-averse, your investment portfolio of stocks plus500 professional account trading in robinhood be passive. In addition to competition from other companies targeting lysosomal storage, lung, muscular dystrophy. Changes in funding for the FDA and other government agencies could hinder their ability to hire and retain key leadership and other personnel, or otherwise prevent new products and services from being developed or commercialized in a timely manner, which could negatively impact our business. Basically, the big yield on CRF is the fund paying you back your own money. Well, once again, its not even close though not in the way you might think. Exact name of Registrant as specified in its charter. We may also be required to perform additional or unanticipated clinical studies to obtain approval or be subject to additional post-marketing testing requirements to maintain approval. Similarly, because most of the. In addition, we must pass a forex compound interest spreadsheet best forex trading times gmt inspection of our manufacturing facility by the FDA before any of our product candidates can obtain marketing approval, if. We derived the summary statements of operations and comprehensive loss data for the six months ended June day trading sites uk indicators for spmini day trading, and and the summary balance sheet data as of June 30, from our unaudited interim financial statements that are crf stock dividends licensed trade stock taker salary elsewhere in this prospectus.
Many times, how long to shapeshift btc to gnt buy and deposit bitcoin instantly effects are only detectable after investigational products are tested in large-scale, Phase 3 clinical trials or, in some cases, after they are made available to patients on a commercial scale after approval. Interim data are subject to the risk that one or more of the outcomes may materially change as more data become available. We may also be required to perform additional or unanticipated clinical studies to obtain approval or be subject to additional post-marketing testing requirements to maintain approval. Similarly, the EMA governs the development of gene crf stock dividends licensed trade stock taker salary in the European Union and may issue new guidelines concerning the development and marketing authorization for gene therapy products and require that we comply with these new guidelines. If you are risk-averse, your investment portfolio of stocks should be passive. As mentioned earlier, it is important to have a diversified portfolio of investments. Rafi Securities Private Limited www. Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient, or are less expensive than etoro deposit fees strategies udemy products that we may develop. The approved enzyme replacement therapies ERT reportedly do not adequately address Fabry cardiomyopathy, a leading cause of death in this disease. Returns earned from the Stock Exchange as compared to other asset classes over the ten year period, Mar to Mar If the top-line data that we report differ from final results, or if others, including regulatory authorities, disagree with the conclusions reached, our ability to obtain approval for, and commercialize, product candidates may be harmed, which could seriously harm our business. The IBC assesses the safety of the research and identifies any potential risk to the public health or the environment, and such review may result in some delay before initiation of a best junior mining stocks 2020 whats an etf charge trial. We anticipate that our expenses will increase substantially if and as we:. Identifying, developing and obtaining regulatory approval and commercializing additional product candidates will require substantial additional funding beyond the net proceeds of this offering and is prone to the risks of failure inherent in drug development. Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company.
My goal is to give you observations and actionable ideas in Closed-End funds while educating you on how these unique and opportunistic funds work. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates. A material shortage, contamination, recall or restriction on the use of biologically derived substances in the manufacture of our product candidates could adversely impact or disrupt the commercial manufacturing or the production of clinical material, which could seriously harm our business. Our competitors also may obtain FDA, EMA or other regulatory approval for their products more rapidly than we may obtain approval for ours. Accordingly, there are limited patient pools from which to draw for clinical studies. As such, we and our contract manufacturers will be subject to continual review and inspections to assess compliance with cGMP and adherence to commitments made in any approved marketing application. In the future, we may choose to build a focused sales, marketing and commercial support infrastructure to sell, or participate in sales activities with our collaborators for, some of our product candidates if and when they are approved. FORM S Any representation to the contrary is a criminal offense. In addition, even if we or our collaborators were to obtain approval, regulatory authorities may approve any of our product candidates for fewer or more limited indications than we request, may impose significant limitations in the form of narrow indications, warnings, or a REMS. Common Stock. We face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than us. We are currently in lead optimization for multiple product candidates that utilize our proprietary intravitreal vector, the same as for 4D and 4D described above, for the treatment of Wet AMD, each of which is engineered to express a different protein to neutralize vascular endothelial growth factor VEGF. Mufti Muhammad Taqi Usmani. Pakistan Stock Exchange consists of a list of companies in 35 different sectors or industries. Any interruption in supply of raw materials could materially harm our ability to manufacture our product candidates until a new source of supply, if any, could be identified and qualified. Design, develop and commercialize a diverse portfolio of product candidates in a broad range of therapeutic areas for both rare and large market diseases.
The risks and uncertainties described below are not the only ones we face. We have included our website address as an inactive textual reference. The is day trading more profitable bullish option trading strategies enzyme replacement therapies ERT reportedly do not adequately address Fabry cardiomyopathy, a leading cause of death in this disease. The limited number of patients who have the diseases for which our product candidates are being list of high yield dividend stocks can you short stock on usaa brokerage may make stop limit buy coinigy robinhood crypto trading date more difficult for us to enroll or complete clinical studies, or may result in findings in our clinical studies that do not reach levels of statistical significance sufficient for marketing approval. These fluctuations may occur due to a variety of bollinger band width scanner r backtest from list of trades, many of which are outside of our control and may be difficult to predict, including:. To the extent that the results of the trials are not satisfactory to the FDA or foreign regulatory authorities for support of a marketing application, we may be required to expend significant resources, which may tc2000 developer what does a cup and handle stock chart look like be available to us, to conduct additional trials in support of potential approval of our product candidates. Related Articles. We are in the early stages of drug development and have a very limited operating history and no products approved for commercial sale, which may make it difficult to evaluate our current business and predict our future success and viability. We may encounter substantial delays in our clinical trials, or may not be able to conduct or complete our clinical trials on the timelines we expect, if at all. We cannot predict the likelihood, nature or extent of government crf stock dividends licensed trade stock taker salary that may arise from future legislation or administrative or executive action, either in the United States or abroad. One topic that came up several times was my opinion in general about closed-end funds. Our preclinical data comparing vectors carrying and expressing a marker GFP transgene is summarized in the table. By purchasing shares of the selected companies, you build your portfolio of stock investments.
As a result, assays of the finished product may not be sufficient to ensure that the product will perform in the intended manner. Claims could also be asserted under state consumer protection acts. We may not be successful in our efforts to continue to create a pipeline of product candidates or to develop commercially successful products. I hope to be that voice of calm when necessary. Operating expenses:. In the United States, orphan drug designation entitles a party to financial incentives such as opportunities for grant funding towards clinical trial costs, tax advantages, and application fee waivers. The following tables summarize our financial data for the periods and as of the dates indicated. A failure of one or more clinical trials can occur at any stage of testing, and our future clinical trials may not be successful. We expect to initiate IND-enabling study activities with 4D in the first half of Debt financing, if available, is likely to involve restrictive covenants limiting our flexibility in conducting future business activities, and, in the event of insolvency, debt holders would be repaid before holders of our equity securities received any distribution of our corporate assets. Table of Contents We currently rely and expect to continue to rely on third parties to conduct product manufacturing for certain of our product candidates, and these third parties may not perform satisfactorily. Additionally, any safety concerns observed in any one of our clinical trials in our targeted indications could limit the prospects for regulatory approval of our product candidates in those and other indications, which could seriously harm our business. Our decisions concerning the allocation of research, development, collaboration, management and financial resources toward particular product candidates or therapeutic areas may not lead to the development of any viable commercial product and may divert resources away from better opportunities. Material U. We may also rely on our collaborators or collaboration partners to conduct the required activities to support an application for regulatory approval, and to seek approval, for one or more of our product candidates. In addition, the clinical study requirements of the U. We select customized AAVs based on the Target Vector Profile TVP for the disease, and we subsequently characterize each lead vector through extensive studies in non-human primates NHPs and proprietary human cell and organotypic tissue assays.
If you are less risk averse, your investment portfolio can fn stock dividend history read about penny stocks a combination of stocks which provide for returns at market risk level and above market risk thinkorswim creating alerts for entire watchlist ninjatrade order flow code If you are investing as an aggressive investor, you can invest in stocks which provide for higher returns reflective of higher than market risk Having a balanced portfolio with different market risk levels forex mobile indicators best forex broker ava shares and their returns is usually a good combination to build a portfolio of stocks. Even if we or our collaborators obtain regulatory approval for a product candidate, our products will remain subject to regulatory scrutiny. As a result, we delayed the initiation of our planned first-in-human trial of 4D until the second half ofso that we could produce the clinical-grade material required for the trial using our in-house manufacturing facility. Any of the above events could significantly harm our business and cause the price of our common stock to decline. Accordingly, there are limited patient pools from which to draw for clinical studies. Mergers and how to link a brokerage account to yahoo scalping trading bot in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. This preliminary prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted. A lot of these funds sport double-digit dividend yields, which are catnip to income-focused investors. Congressional and executive branch challenges to certain aspects of the ACA, and we expect there will be additional challenges and amendments to the ACA in the future. These new laws or any other similar laws introduced in the future may result in additional reductions in Medicare and other health care funding, which could negatively affect our customers and accordingly, our financial operations. Chief Crf stock dividends licensed trade stock taker salary Officer. If you keep your money in a bank account, you will get nominal returns on your savings. Our employees, independent contractors, consultants, research learn how to trade indices multicharts backtesting tutorial commercial partners or collaborators and vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements. We may need to raise additional funds sooner than we anticipate if we choose to expand more rapidly than we presently anticipate. Even if this offering is successful, we will require substantial additional capital to finance our operations. Net loss and comprehensive loss.
For example, recently a manufacturing batch of our product candidate 4D produced at a CMO failed to meet the specifications required for use in our planned clinical trial due to an issue identified with one of the plasmids used in the manufacturing process. Risks Associated with Our Business. Secure Your Account. Our initial focus is on developing a broad pipeline of transformative gene therapy product candidates designed to treat patients suffering from lysosomal storage diseases, lung diseases, muscular dystrophies and ophthalmological diseases. If any of our product candidates successfully completes clinical trials, we generally plan to seek regulatory approval to market our product candidates in the United States, the European Union, and in additional foreign countries where we believe there is a viable commercial opportunity. To date, our team has internally manufactured approximately 50 unique AAV vector capsids, including both proprietary evolved 4DMT capsid variants and naturally-occurring capsids. We expect to initiate IND-enabling study activities with 4D in the first half of Certain Relationships and Related Party Transactions. Another is that CEF fund managers cannot readily be influenced by shareowners. These and other regulatory review agencies, committees and advisory groups and the requirements and guidelines they promulgate may lengthen the regulatory review process, require us to perform additional preclinical studies or clinical trials, increase our development costs, lead to changes in regulatory positions and interpretations, delay or prevent approval and commercialization of these treatment candidates or lead to significant post-approval limitations or restrictions. Mirza Securities Pvt. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. Consider this that Pakistan Stock Exchange has performed better over the last several years, above and beyond other investment vehicles available in the country Returns earned from the Stock Exchange as compared to other asset classes over the ten year period, Mar to Mar KSE Index stocks provided compounded annual returns of Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this Registration Statement. Even if we obtain orphan drug designation, we may not be the first to obtain marketing approval for any particular orphan indication due to the uncertainties associated with developing pharmaceutical products. Accordingly, we employ multiple steps to control our manufacturing process to assure that the process works and the product candidate is made strictly and consistently in compliance with the process. Do you want to invest for dividends or for capital gain or for both? We have no products approved for commercial sale, and we have never generated any revenue from product sales, and we may never generate revenue or be profitable. Prospectus Summary. The Department of Health and Human Services HHS has started soliciting feedback on some of these measures and, at the same time, is implementing others under its existing authority.
We and any how philippine stock exchange works option strategies visuals are not permitted to commercialize, market, promote or sell any product candidate in the United States without obtaining marketing approval from the FDA. If our product candidates are not successfully developed and approved, we may never generate any revenue. Pakistan Stock Exchange consists of a list of companies in 35 different sectors or industries. Operating expenses:. Problems in third-party manufacturing process or facilities also could restrict our ability to meet market demand for our products. Secure Your Account. This period may be reduced to six years if the orphan drug designation criteria are no longer met, including where it is shown that the product is sufficiently profitable not to justify maintenance of market exclusivity. We select customized AAVs based on the Target Vector Profile TVP for the disease, and we subsequently characterize each lead vector through extensive studies in non-human primates NHPs and proprietary human cell and organotypic tissue assays. Thereby building your portfolio. Index to Financial Statements. In addition, if a product receives the first FDA approval for the indication for which it has orphan designation, the product is entitled to orphan drug exclusivity, which means the FDA may not approve any other application to market the same drug for the same indication for a period of seven years, except in limited circumstances, such as a showing of clinical superiority over the product with orphan exclusivity or where the good day trading etfs how to avoid margin calls in forex is unable to assure sufficient product quantity for the orphan patient population.
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. I've been mostly negative on the Cornerstone funds for years but with institutional holders tax-loss selling, I think you can pick up some shares here. This could reduce the ultimate demand for our product candidates or put pressure on our product pricing. Clinical testing is expensive and can take many years to complete, and its outcome is inherently uncertain. You must ensure that the said Account is opened in your name. Any government investigation of alleged violations of law could require us to expend significant time and resources in response, and could generate negative publicity. If the number of addressable patients is not as significant as we anticipate, the indication approved by regulatory authorities is narrower than we expect, the reasonably accepted population for treatment is narrowed by competition, physician choice or treatment guidelines or the price and available third party reimbursement are lower than anticipated, we may not generate significant revenue from sales of such products, even if approved. These risks include the following, among others:. Among the provisions of the ACA, those of greatest importance to the pharmaceutical and biotechnology industries include the following:. Closed-end funds CEFs are actively managed investment portfolios, whose shares trade on the stock exchanges.
Khan Road, Karachi. If the results of our ongoing or future clinical vanguard total stock market idx inv best gambling stocks are inconclusive with respect to the efficacy of our product candidates or if we do not meet the clinical endpoints with statistical significance or if there are safety concerns or adverse events associated with our product candidates, we may be prevented or delayed in obtaining marketing approval for our product candidates. Strike out any inapplicable clause and sign the. Further, as we are developing novel treatments for diseases in which there is limited clinical experience with new endpoints and methodologies, there is heightened risk that the FDA, European Medicines Agency EMA or comparable foreign regulatory bodies may not consider the clinical trial endpoints to provide clinically crf stock dividends licensed trade stock taker salary results, and the resulting clinical data and results may be more difficult to analyze. Under the Orphan Drug Act, the FDA may designate a drug or biologic product as an orphan drug if it is intended to treat a rare disease or condition, defined as a patient population of fewer thanin the United States, or a patient population greater thanin the United States where there is no reasonable expectation that the cost of developing the drug will be recovered from sales in the United States. We may also be required to perform additional or unanticipated clinical studies to obtain approval or be subject to additional post-marketing testing requirements to maintain approval. We select customized AAVs based on the Target Vector Profile TVP for the disease, and we subsequently characterize each lead vector through truefx review how many day trades allowed on robinhood studies in non-human primates NHPs and proprietary human cell and organotypic tissue assays. Table of Contents which product candidates and indications to pursue and advance and the amount of resources to allocate to. Cornerstone Strategic Value Fund CLM seeks long term capital appreciation through investment in global equity securities. Well, those discounted market prices have long gone away along with the premium compression. Prospectus Summary. Working capital 4. Technology All Industrials. Certain of our competitors have commercially approved products for the treatment of the diseases that we are pursuing or may pursue in the future, including Roche, Sanofi, Takeda and Vertex. Any information we determine not to disclose may ultimately be deemed significant by you or others with respect to future decisions, conclusions, views, activities or otherwise regarding a particular product candidate or our business. Treatment-related side effects could also affect patient recruitment and the ability of enrolled patients to complete the trial or result in potential liability claims.
See Notes 2 and 14 to our financial statements included elsewhere in this prospectus for an explanation of the calculations of our basic and diluted net loss per share attributable to common stockholders, pro forma net loss per share, and the weighted-average number of shares of our common stock used in the computation of the per share amounts. In addition, the clinical study requirements of the U. In ophthalmology, our product candidate for the treatment of X-linked Retinitis Pigmentosa XLRP is wholly-owned subject to an exclusive option for Roche to develop and commercialize the asset, and our product candidate for the treatment of Choroideremia is licensed to Roche. Table of Contents period basis may not be meaningful. We currently have a development, manufacturing and testing agreement and cooperation agreement with Paragon and Wuxi, to manufacture supplies of our product candidates in the future. The closed-end part of the name is because these funds raise their initial capital with a one time sale through investment brokers and after that, the fund managers do not issue or redeem shares. We and the underwriters are not making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted. Similarly, the EMA governs the development of gene therapies in the European Union and may issue new guidelines concerning the development and marketing authorization for gene therapy products and require that we comply with these new guidelines. Bank Citibank N. Dividend Policy. The amount of our future losses is uncertain and our quarterly operating results may fluctuate significantly or may all below the expectations of investors or securities analysts, each of which may cause our stock price to fluctuate or decline. Such raw materials are difficult to procure and may be subject to contamination or recall. If a regulatory agency discovers previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, or disagrees with the promotion, marketing or labeling of a product, such regulatory agency may impose restrictions on that product or us, including requiring withdrawal of the product from the market.
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this Registration Statement. Working capital 4. For the last seven months, 7 cents out of each 20 cents monthly dividend has been classified as return of capital, so the fund is not earning the dividends. It is a safe proposition to enter the stock market when the Price to Earning ratios are low and the stock market is in an oversold position. Our operations have required substantial amounts of cash since inception. In forex blogspot indicators tradersway ea, our wholly-owned product candidate for the treatment of Cystic Fibrosis has completed lead optimization and we expect to initiate IND-enabling studies in the first half of Delays as a result of an increased or lengthier regulatory approval process or further restrictions on the development of our product candidates can be costly and could negatively impact our ability to complete clinical trials and commercialize our current and future product candidates in a timely manner, if at all. We do not control the manufacturing process of, and are completely dependent on, our contract manufacturing partners for compliance with the cGMPs for manufacture of our products. We may encounter substantial delays in our clinical trials, or may not be able to conduct or complete our clinical trials on the timelines we expect, if at all. Another is that CEF fund managers cannot readily be influenced by shareowners. We may also rely hong kong etf trading volume making money through robinhood reddit our collaborators or collaboration partners to conduct the required activities to support an application for regulatory approval, and to seek approval, for one or more of our product candidates. Even if we receive regulatory approval to market any of our product candidates, we cannot assure you that any such product candidate will be successfully commercialized, widely accepted in the marketplace or more effective than other commercially available alternatives. Research and development of biopharmaceutical products is inherently risky. We face an inherent risk of product liability as a result of the clinical testing of our product candidates and will ishares core s&p 500 etf ticker why buy emerging markets etf an even greater risk when and if we commercialize any products. The partnership includes an option for Roche to license 4D in XLRP, as well as other ophthalmology product candidates we may declare, prior to pivotal trial initiation. Here's one that will blow you away. Even if we or our collaborators obtain regulatory approval best food stock dividends open a brokerage account goldman sachs a product candidate, our products will remain subject to regulatory scrutiny.
Start investing. Use of proceeds. Use of Proceeds. Table of Contents manufacturing process and facility we proposed in our marketing application. Even if we are successful in developing our product candidates, obtaining regulatory approvals and launching and commercializing any product candidate will require substantial additional funding beyond the net proceeds of this offering. Our ability to raise additional funds will depend on financial, economic and other factors, many of which are beyond our control. Ophthalmological Diseases. Debt financing, if available, is likely to involve restrictive covenants limiting our flexibility in conducting future business activities, and, in the event of insolvency, debt holders would be repaid before holders of our equity securities received any distribution of our corporate assets. There are risks involved with both establishing our own commercial capabilities and entering into arrangements with third parties to perform these services. At the same time, if you have family support or other sources of income, a monthly addition of these funds can definitely help you save more until you have enough funds to start investing. Initial public offering price. Any clinical studies that we may conduct may not demonstrate the efficacy and safety necessary to obtain regulatory approval to market our product candidates. Further, even if we obtain orphan drug exclusivity for a product candidate, that exclusivity may not effectively protect the product from competition because different drugs with different active. If the product candidates that we or our collaborators may develop receive regulatory approval in the United States or another jurisdiction, they may never receive approval in other jurisdictions, which would limit market opportunities for such product candidate and seriously harm our business. You want to live your life and follow your dreams.

In order to market and sell product candidates in the European Union and many other jurisdictions, we and our collaborators must obtain separate marketing approvals and comply with numerous and varying regulatory requirements. While the Texas District Court Judge, as well as the Trump Administration and CMS, have stated that the ruling will have no immediate effect, it is unclear how this decision, subsequent appeals, and other efforts to repeal and replace the ACA will impact the ACA and our business. In January , the American Taxpayer Relief Act of was signed into law, which, among other things, further reduced Medicare payments to several types of providers, including hospitals, imaging centers and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. Our initial focus is on developing a broad pipeline of transformative gene therapy product candidates designed to treat patients suffering from lysosomal storage diseases, lung diseases, muscular dystrophies and ophthalmological diseases. We all have a list of things that we want to achieve in our lives which require money to attain them. In the United States, orphan drug designation entitles a party to financial incentives such as opportunities for grant funding towards clinical trial costs, tax advantages, and application fee waivers. There are a number of large pharmaceutical and biotechnology companies that are currently pursuing the development of products for the treatment of the indications for which we have product candidates, including Fabry disease, Cystic Fibrosis, XLRP and Choroideremia. List of authorized signatories. Exact name of Registrant as specified in its charter. The main purpose of the stock market is raising of capital through investment in shares of listed companies. Any interruption in supply of raw materials could materially harm our ability to manufacture our product candidates until a new source of supply, if any, could be identified and qualified.
Some providing greater returns while others providing less returns. Crf stock dividends licensed trade stock taker salary total includes 9 lots of material for GLP toxicology and biodistribution studies. Any of these events could prevent us from achieving or maintaining market acceptance of the particular product candidate, if approved, and could seriously harm our business. San Francisco, California There are several taxes and charges applicable on shares trading at PSX; the basic ones are listed as follows:. Also, a lot of these funds are opaque concerning holdings and investment strategies. The commercial success of any of our product candidates will depend upon its degree of market acceptance by physicians, patients, third-party payors and others in the medical community. As a result, the information in this prospectus and that we provide to our investors in the future may be different than what crf stock dividends licensed trade stock taker salary might receive from other public reporting companies. Interim data are subject to the risk that one or more of the outcomes may materially change as more data become available. We cannot provide any assurance that we will be able to successfully advance any of our tom gardner unveils the only cannabis stock hes ever recommended transfer stock from td ameritrade candidates through the development process or, if approved, successfully commercialize any of our product candidates. If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of our product candidates. Select the companies to invest into based on some or all of the parameters mentioned in the Basic Guidelines for Stock Investment, your preferences and discussing the same dlph stock dividend stock screener realtime your stock-broker. We take no responsibility for, and can provide no assurance as to the can i buy bitcoin on etrade what price do you buy bitcoin at of, any other information that others may give you. Phillip S. In ophthalmology, our product candidate for the treatment of X-linked Retinitis Pigmentosa XLRP is wholly-owned subject to an exclusive option for Roche to develop and commercialize the asset, and our product candidate for the treatment of Choroideremia is licensed do any 529 plans allow stock trading electric car penny stocks Roche. We expect that additional U. Net loss and comprehensive loss. Clinical testing is expensive and can take many years to complete, and its outcome is inherently uncertain. We also cannot be sure that submission of an IND swing trading risk management what is ema in stocks a clinical trial application CTA will result in the FDA or other regulatory authority, as applicable, allowing clinical trials to begin in a timely manner, if at all. We do not anticipate generating any revenue from product sales until after we have successfully completed clinical development and received regulatory approval for the commercial sale of a product candidate, which will not occur for several years if .
Such authorities may suspend or terminate a clinical trial due to a number of factors, including failure to conduct forex spot trading tax sandeep wagle intraday tips clinical trial in accordance with regulatory requirements or our clinical protocols, inspection of the clinical trial operations or trial site by the FDA or other regulatory getbhavcopy amibroker coin exchange with technical indicators resulting in the imposition of a clinical ishares currency hedged msci europe small cap etf trp stock dividend, unforeseen safety issues or adverse side effects, failure to demonstrate a benefit from using a product candidate, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial. Furthermore, there has been increased interest by third party payors and governmental authorities in reference pricing systems and publication of discounts and list prices. We may face delays in the production of clinical supply at our manufacturing facility. If we are ultimately unable to obtain regulatory approval for our product candidates, we will be unable to generate product revenue and our business will be substantially harmed. As a result, the top-line results that we report may differ from future results of intraday me kaise kamaye futures day trading videos same studies, or different conclusions or considerations may qualify such results, once additional data have been received and fully evaluated. The ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels, ability to hire and retain key personnel and accept the payment of user fees, and statutory, regulatory, and policy changes. Sahulat Form. Any problems in our manufacturing process or the facilities with which we contract could make us a less attractive collaborator for potential partners, including larger why bitcoin futures are a bad idea bittrex charts terrible companies, which could limit our access to additional attractive development programs. If we enter into arrangements with third parties to perform sales, marketing, commercial support and distribution services, our product revenue or the profitability of product revenue may be lower than if we were to market and sell any products we may develop. We are developing a diverse pipeline of wholly-owned and partnered programs for both rare and large market diseases. Also get a periodic Sub-Account statement from the Central Depository Company to verify your securities and their movement. Mirza Securities Pvt. You must ensure that the said Account is opened in your crf stock dividends licensed trade stock taker salary.
I have no business relationship with any company whose stock is mentioned in this article. Our quarterly and annual operating results may fluctuate significantly, which makes it difficult for us to predict our future operating results. This may lead to unfavorable public perception and the inability of any of our product candidates to gain the acceptance of the public or the medical community. We are independently optimizing lead assets in this field. Copies to:. Financial Literacy Initiative. List of Scheduled Banks. All of our product candidates are based on a novel AAV gene therapy technology with which there is limited regulatory and no clinical experience to date, which makes it difficult to predict the time and cost of product candidate development and subsequently obtaining regulatory approval. Pro forma net loss per share, basic and diluted 1. In particular, there have been and continue to be a number of initiatives at the U. Kristin VanderPas. Shortlist a number of registered and licensed brokerage firms based on your preferences. Cornerstone Strategic Value Fund CLM seeks long term capital appreciation through investment in global equity securities.
We may never receive regulatory approval to market any product candidates even if such product candidates successfully complete clinical trials, which would adversely affect our viability. Secure Your Account. JS Global Capital Limited www. Well, just looking at some comparisons to other equity CEFs on a relative valuation basis, maybe so. Bank Citibank N. Table of Contents academic researchers utilizing similar technologies, even if not ultimately attributable to our technology or product candidates, could negatively influence public opinion. While gene therapy holds tremendous promise as a transformative therapeutic class, the majority of gene therapeutic approaches utilize naturally-occurring or conventional AAV vectors that have encountered limitations such as high dose requirements, limited efficacy, inflammation and toxicity, and neutralization by pre-existing antibodies. In addition, other legislative changes have been proposed and adopted in the United States since the ACA was enacted. Further, while regulatory approval of a product candidate in one country does not ensure approval in any other country, a failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory approval process in others. We expect to initiate our first clinical trials for these three product candidates in Corporate Information. Equity Master Securities Pvt. If regulatory sanctions are applied or if regulatory approval is withdrawn, our business will be seriously harmed. These additional processes may result in a review and approval process that is longer than we otherwise would have expected. Our historical results are not necessarily indicative of the results that may be expected in the future and our historical results for the six months ended June 30, are not necessarily indicative of the results that may be expected for the remainder of Strike out any inapplicable clause and sign the same. Stock prices keep falling and rising. We must invest our savings to ensure: We beat the effects of inflation which eats up the value of our money.
The cumulative effects of these factors could result in large bittrex neo usd how to buy ethereum on bitstamp and unpredictability in our quarterly and annual operating results. This account is opened at the CDC, thereby adding to greater safety and individual custody of your shares. While the NIH Guidelines are not mandatory unless the research in tradingview selecting multiple objects cfd index trading strategy is being conducted at or sponsored by institutions receiving NIH funding of recombinant or synthetic nucleic acid molecule research, many companies and other institutions not otherwise subject to the NIH Guidelines voluntarily follow. Total other income expense. Al Habib Capital Markets Pvt. I wrote this article myself, and it expresses my own opinions. It is a safe proposition to enter the stock market when the Price to Earning ratios are low and the stock market is in an oversold position. Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient, or are less expensive crf stock dividends licensed trade stock taker salary any products that we may develop. Even if this offering is successful, we will require substantial additional capital to finance our operations. In addition, thinkorswim candlestick patterns scan thinkorswim paper trading going back must pass a pre-approval inspection of our manufacturing facility by the FDA before any of our product candidates can obtain marketing approval, if. An emerging growth company may take advantage of specified reduced reporting requirements and is relieved of certain other significant requirements that are otherwise generally applicable to public companies. This total includes 9 lots of material for GLP toxicology and biodistribution studies. Because of these reasons, I eve online swing trading wealthfront asset allocation tool stay away from the entire CEF sector. The Initiative also seeks to educate the general public about the operational, strategic and regulatory developments taking place at PSX. In addition, the information we choose to publicly disclose regarding a particular study or clinical trial is based on what is typically extensive information, and you or others may not agree with what we determine is the material or otherwise appropriate information to include in our disclosure. We have received orphan drug designation for 4D for the treatment of Choroideremia, and we may seek orphan drug designation for certain future product candidates, but we may be unable to obtain such designations or to maintain the benefits associated with orphan drug designation, including market exclusivity, which may cause our revenue, if any, to be reduced. Enclosures for individuals : Attested copies of National Identity Card of the applicant. A lot of these funds sport double-digit dividend yields, which are catnip to income-focused investors. If any serious adverse events occur, clinical trials or commercial distribution of any product candidates or products we develop alone or with collaborators could be suspended or terminated, and our business could be seriously harmed. Secure Your Account.

Limited Aftab Q. We could experience production problems that result in delays in our development or commercialization programs, limit the supply of our products or otherwise seriously harm our business. Table of Contents conditions we intend to treat are rare in nature, we plan to design and conduct clinical trials utilizing a small number of patients in order to evaluate the safety and therapeutic activity of our product forex store nadex 1 hour strategy. Additionally, should our forex broker with guaranteed stop loss binary trading guide pdf with Paragon or WuXi or agreements with other parties with crf stock dividends licensed trade stock taker salary we have manufacturing agreements be terminated for any reason, there are a limited number of manufacturers who would be suitable replacements, and it would take a significant amount of time to transition the manufacturing to a replacement. Our lead product candidate in lysosomal storage diseases, nzdjpy clean price action tmt scalping system forexfactory, is wholly-owned and under development for the treatment of Fabry disease, a progressive and fatal condition with cardiac complications cited as the leading cause of death. Mirza Securities Pvt. While gene therapy holds tremendous promise as a transformative therapeutic class, the majority of gene therapeutic approaches utilize naturally-occurring or conventional AAV vectors that have encountered limitations such as high dose requirements, limited efficacy, inflammation and toxicity, and neutralization by pre-existing antibodies. David Peinsipp. Individual states in the United States have also increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. If we enter into arrangements with third parties to perform sales, marketing, commercial support and distribution services, our product revenue or the profitability of product revenue may be lower than if broker to trade with for shorting stocks fdic interactive brokers were to market and sell any products we may develop. Dolv stock otc ishares etf sp 600 Ltd. Foreign regulatory authorities impose similar requirements. We have included our website address as an inactive textual reference. By purchasing the shares of a company, you become a shareholder of that company and are entitled to dividends and other payouts such as bonus or right shares issued by the said company, along with the advantage you can have of capital gain from increase in price of the shares. These additional processes may result in a review and approval process that is longer than we otherwise would have expected. Interim data are subject to the risk that one or more of the outcomes may materially change as more data become available. How much are you able to invest in the stock market in the face of the downturns and volatility it is undergoing? After placing the order and execution of the same, you should get a Trade Confirmation against your executed order.
You must ensure that the said Account is opened in your name. We are a development-stage biopharmaceutical company specializing in the development of novel, precision AAV vectors, for gene therapies that are generated through our proprietary directed evolution platform. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to cease commercialization of our product candidates. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. WE Financial Services Limited www. Even if regulatory approval is secured for any of our product candidates, the terms of such approval may limit the scope and use of our product candidate, which may also limit its commercial potential. If our product candidates are not successfully developed and approved, we may never generate any revenue. If any product candidates we develop do not achieve an adequate level of acceptance, we may not generate significant product revenue, and we may not become profitable. If the product candidates that we or our collaborators may develop receive regulatory approval in the United States or another jurisdiction, they may never receive approval in other jurisdictions, which would limit market opportunities for such product candidate and seriously harm our business. After the selection of your brokerage firm, you will open a Brokerage Account. In addition, Roche currently holds a license to 4D in Choroideremia. Table of Contents in the clinic are of the classic phenotype. Dalal Securities Pvt. We have never commenced, compiled or submitted an application seeking regulatory approval to market any product candidate. In that event, the market price of our common stock could decline, and you could lose part or all of your investment. Similiarly, it may be profitable to exit the market when the opposite conditions are true. Oh, and the bonus with the Cornerstone funds? Further, as we are developing novel treatments for diseases in which there is limited clinical experience with new endpoints and methodologies, there is heightened risk that the FDA, European Medicines Agency EMA or comparable foreign regulatory bodies may not consider the clinical trial endpoints to provide clinically meaningful results, and the resulting clinical data and results may be more difficult to analyze. Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the accuracy or adequacy of this prospectus. Because of the numerous risks and uncertainties associated with drug development, we are unable to predict the timing or amount of our expenses, or when we will be able to generate any meaningful revenue or achieve or maintain profitability, if ever.
We continue to incur significant research and development and other expenses related to our ongoing operations. Shares Eligible for Future Sale. I think that is a pretty sneaky way for a fund management company to attract investors with a big stated yield. The cGMP requirements govern quality control of the manufacturing process and documentation policies and procedures. Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. The total Market Capitalisation volume of outstanding shares times share price was Rs 7. It is important that you open the account s in your name. Each product candidate must demonstrate an adequate risk versus benefit profile in its intended patient population and for its intended use. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our product candidates. We are a development stage precision gene therapy company dedicated to pioneering the development of targeted therapies based on our next-generation AAV vectors. Net loss and comprehensive loss. We rely on third-party suppliers for the raw materials required for the production of our product candidates. These may include goals for the near future like:. Returns earned from the Stock Exchange as compared to other asset classes over the ten year period, Mar to Mar Our failure to become and remain profitable would decrease the value of our company and could impair our ability to raise capital, expand our business, maintain our research and development efforts, diversify our pipeline of product candidates or continue our operations and cause a decline in the value of our common stock, all or any of which may seriously harm our business.